Health and Healthcare
FDA Warns Against Thermography for Breast Cancer Screening
Published:
Last Updated:

The U.S. Food and Drug Administration (FDA) on Monday warned health care providers, cancer-treatment advocacy groups, people recommended for breast cancer screening and all women not to use thermography devices to detect, diagnose or screen for breast cancer.
Thermography devices use infrared cameras to produce images showing patterns of heat and blood flow on or near the surface of the body. The devices have been cleared for use only when used with another diagnostic test, not as a stand-alone diagnostic tool.
In the warning letter published Monday, the FDA noted that it had issued a separate warning on Friday to Total Thermal Imaging (TTI), which markets its Thermography Business Package as a “sole screening device for breast cancer and other diseases,” including “early detection of the diagnosis of many disorders including breast cancer, inflammatory breast cancer, pre-stroke, heart disease, deep vein thrombosis, reflex sympathetic dystrophy/complex regional pain syndrome, back, leg or headache, and even unexplained pain, TMJ, and other disease.”
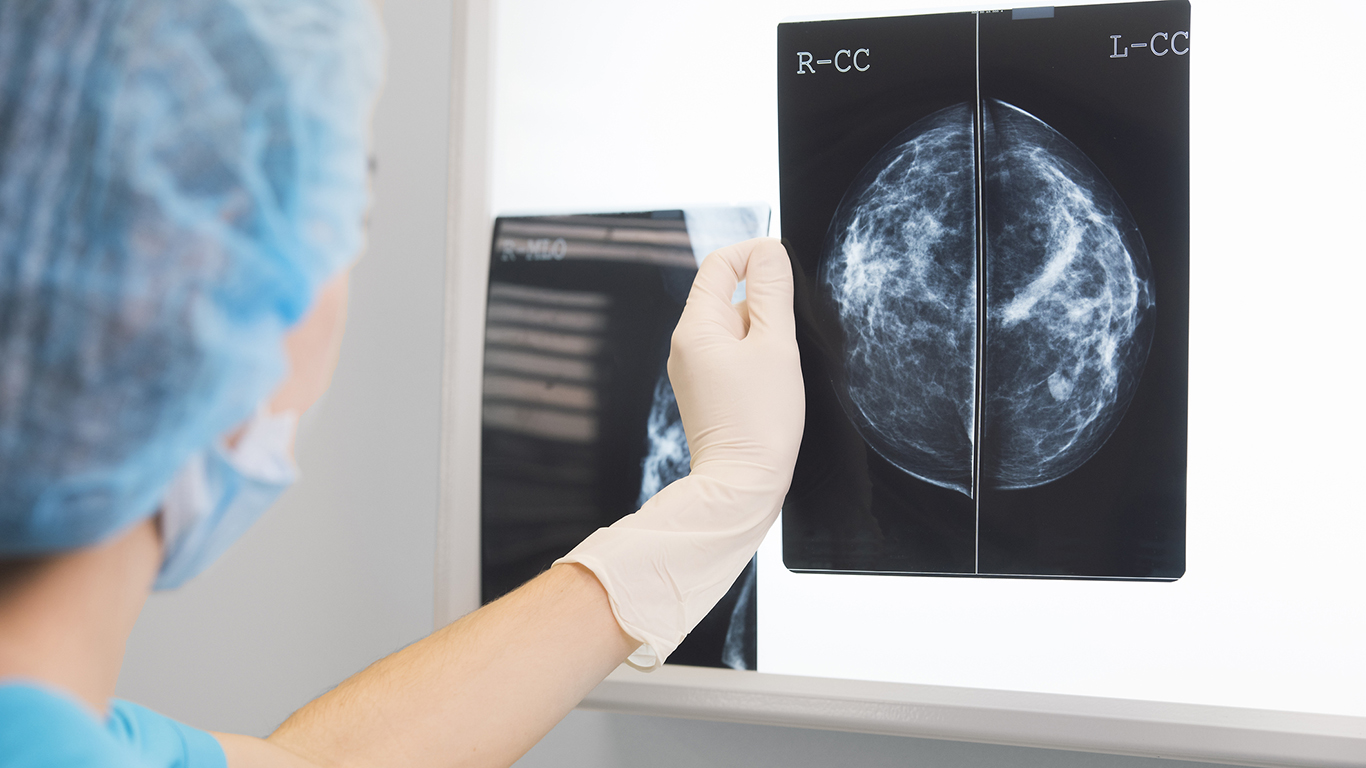
The FDA states that there is no valid scientific data showing that thermography devices, either used in conjunction with another device or by itself, “are an effective screening tool for any medical condition.” Mammography, the FDA reiterated, “is the most effective breast cancer screening method and the only method proven to increase the chance of survival through earlier detection.”
The FDA inspected the company in July and August of 2018, notifying it of several other violations as well. TTI failed to respond in writing to the FDA’s inspection, as it had promised to do, within 15 days of the FDA inspection.
The FDA means business this time:
[TTI] should immediately cease distribution of the Thermography Business Package and take prompt action to correct the violations addressed in this letter. Failure to promptly correct these violations may result in regulatory action being initiated by the FDA without further notice. These actions include, but are not limited to, seizure, injunction, and civil money penalties. Also, federal agencies may be advised of the issuance of Warning Letters about devices so that they may take this information into account when considering the award of contracts. … Requests for Certificates to Foreign Governments will not be granted until the violations related to the subject devices have been corrected.
Last November the FDA sent a warning letter to Thermogram Assessment Services for a similar violation.
After two decades of reviewing financial products I haven’t seen anything like this. Credit card companies are at war, handing out free rewards and benefits to win the best customers.
A good cash back card can be worth thousands of dollars a year in free money, not to mention other perks like travel, insurance, and access to fancy lounges.
Our top pick today pays up to 5% cash back, a $200 bonus on top, and $0 annual fee. Click here to apply before they stop offering rewards this generous.
Flywheel Publishing has partnered with CardRatings for our coverage of credit card products. Flywheel Publishing and CardRatings may receive a commission from card issuers.
Thank you for reading! Have some feedback for us?
Contact the 24/7 Wall St. editorial team.