Chimerix Inc. (NASDAQ: CMRX) has become the latest company to get an Ebola announcement out. With scares rising in America after last week’s disclosure of a confirmed Ebola case in Dallas, Texas, Ebola drug treatments offer rather large implied upside if there is a potential treatment. The one issue we would note is that this is not Chimerix’s first announcement around Ebola and highly infectious diseases and viruses.
Chimerix has announced that brincidofovir has been provided for potential use with patients with Ebola virus disease. These requests were made by treating physicians. Emergency Investigational New Drug Applications (EIND) were granted by the U.S. Food and Drug Administration (FDA). This is not the first indication for brincidofovir in the fight against rare infectious diseases and viruses.
Chimerix said in its press release that the company is working closely with the FDA to finalize a clinical trial protocol early this week to assess the safety, tolerability and efficacy of brincidofovir in patients who are confirmed to have an infection with the Ebola virus. Also, additional tests of brincidofovir in in vivo (animal) models of Ebola virus infection are currently underway.
ALSO READ: 8 IPOs Coming This Week
The company said:
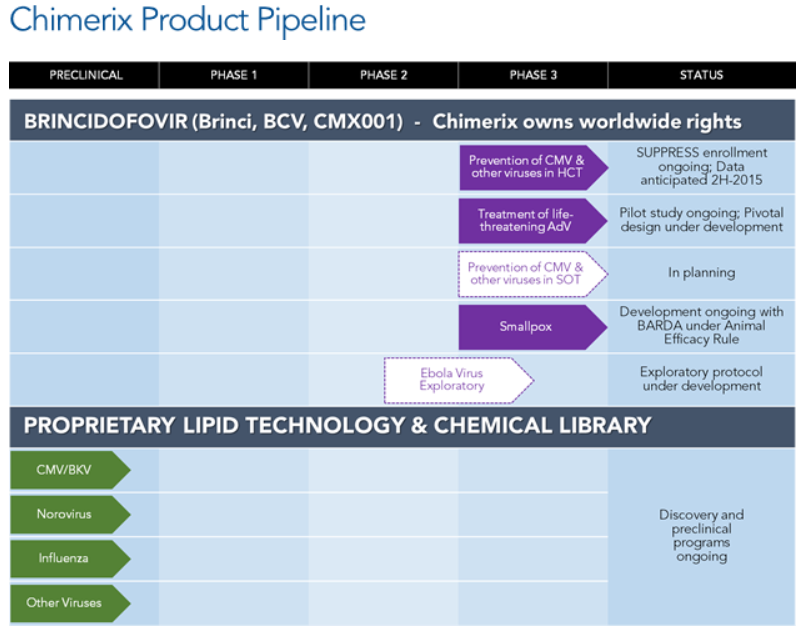
Chimerix is committed to working with global health organizations and government agencies in the fight against the Ebola virus outbreak. Based on in vitro data from work conducted by the CDC and the National Institutes of Health suggesting brincidofovir’s activity against Ebola, we are hopeful that brincidofovir may offer a potential treatment for Ebola Virus Disease during this outbreak. Data collected over years of clinical development of brincidofovir have allowed us to progress this compound into Phase 3 programs for cytomegalovirus and adenovirus infections, and provided information on the safety and dosing of brincidofovir to allow it to be explored as a potential therapy for Ebola Virus Disease.
Brincidofovir is Chimerix’s lead product candidate. This is an oral nucleotide analog that has, according to Chimerix, shown broad-spectrum in vitro antiviral activity against all five families of DNA viruses that affect humans, including viruses in the herpes virus family and adenovirus. Chimerix said in its release that it is also working with the Biomedical Advanced Research and Development Authority (BARDA) to develop brincidofovir as a medical countermeasure against smallpox. Brincidofovir has received Fast Track designation from the FDA for cytomegalovirus, adenovirus and smallpox.
While this company has a $1.1 billion market cap, it may be a while before any revenues are seen out of it. Few analysts follow Chimerix, and the consensus revenue estimates are too small to bother noting for 2014 and 2015. That being said, one analyst does at least see $80 million in total company revenues for 2015 — against a consensus of $12.5 million.
ALSO READ: 7 Must-Know Earnings Season Surprises
Chimerix shares were up 4% at $31.31 shortly after the open on Monday. Note though that the stock was up as high as $32.76 on the news earlier, which also made for a new 52-week high. Below is an image of the company’s drug pipeline.
Are You Ahead, or Behind on Retirement?
If you’re one of the over 4 Million Americans set to retire this year, you may want to pay attention. Many people have worked their whole lives preparing to retire without ever knowing the answer to the most important question: am I ahead, or behind on my goals?
Don’t make the same mistake. It’s an easy question to answer. A quick conversation with a financial advisor can help you unpack your savings, spending, and goals for your money. With Zoe Financial’s free matching tool, you can connect with trusted financial advisors in minutes.
Why wait? Click here to get started today!
Thank you for reading! Have some feedback for us?
Contact the 24/7 Wall St. editorial team.