Health and Healthcare
Who's to Blame for the US Opioid Epidemic
Published:
Last Updated:

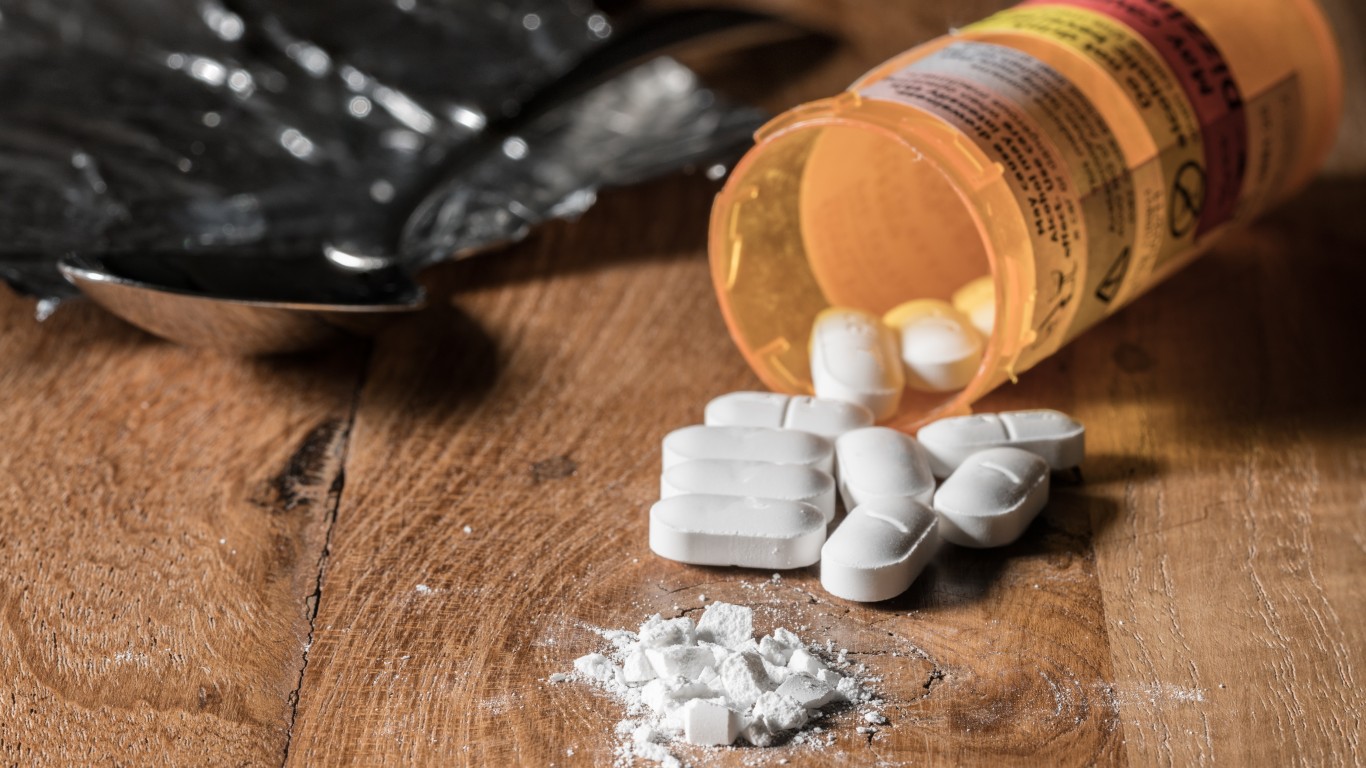
For more than half a decade, the United States has seen an incredible rise of an opioid epidemic within its borders. Fatal overdoses have claimed the lives of many as the result of rampant and unwarranted prescriptions. transmucosal immediate-release fentanyl (TIRF) drugs make up a significant piece of this drug pie. According to a recent study, drug makers and federal regulators are the ones to blame for this crisis.
Note that TIRF drugs are approved for use in cancer patients who’ve developed tolerance against the around-the-clock opioids normally used to treat their pain.
As many as 55% of patients prescribed TIRFs did not meet these criteria and should never have been prescribed them, according to a new report published in the Journal of American Medical Association.
In a statement responding to the study, the U.S. Food and Drug Administration (FDA) said it “shares the concerns about how TIRF products are being used and whether the Risk Evaluation and Mitigation Strategy (REMS) program is working as intended.”
However, this misuse had revealed itself between 2012 and 2017, but the drug makers failed to report any errant prescribers to the FDA, and no prescribers had their special certification revoked. In 2017, the U.S. opioid epidemic claimed the lives of nearly 50,000 through fatal overdoses.
Dr. Caleb Alexander, co-director of the Center for Drug Safety and Effectiveness at the Johns Hopkins Bloomberg School of Public Health, commented:
What’s alarming is it’s taken so long. Serious deficiencies in the program have been known for some time, and the pace of change has not been faster. We’re more than six years out from the program’s inception, and there have been many opportunities that have been missed on the part of opioid manufacturers and [the] FDA.
The FDA held a public advisory committee meeting on the matter in August 2018, and “has been actively assessing the recommendations of our advisory committee on the effectiveness of the REMS and necessary changes,” the FDA statement says.
The average American spends $17,274 on debit cards a year, and it’s a HUGE mistake. First, debit cards don’t have the same fraud protections as credit cards. Once your money is gone, it’s gone. But more importantly you can actually get something back from this spending every time you swipe.
Issuers are handing out wild bonuses right now. With some you can earn up to 5% back on every purchase. That’s like getting a 5% discount on everything you buy!
Our top pick is kind of hard to imagine. Not only does it pay up to 5% back, it also includes a $200 cash back reward in the first six months, a 0% intro APR, and…. $0 annual fee. It’s quite literally free money for any one that uses a card regularly. Click here to learn more!
Flywheel Publishing has partnered with CardRatings to provide coverage of credit card products. Flywheel Publishing and CardRatings may receive a commission from card issuers.
Thank you for reading! Have some feedback for us?
Contact the 24/7 Wall St. editorial team.