 In the last four months, Dendreon Corp. (DNDN) has shed almost 84 percent in market value as disgruntled investors washed hands due to disappointing sales of prostate-cancer drug Provenge. Contrary to the growing growls of Wall Street bears, the once high-flying biotech company is in little danger of running out of money.
In the last four months, Dendreon Corp. (DNDN) has shed almost 84 percent in market value as disgruntled investors washed hands due to disappointing sales of prostate-cancer drug Provenge. Contrary to the growing growls of Wall Street bears, the once high-flying biotech company is in little danger of running out of money.
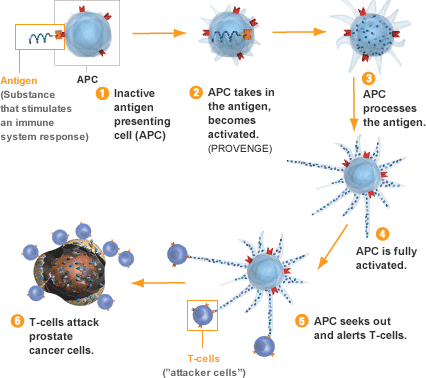
Approved in April 2010 for the treatment of advanced (hormone-refractory) prostate cancer, Provenge is the first therapy in the U.S. designed to induce the body’s own immune system to attack prostatic tumor cells. Management’s initial forecast that 2011 sales could hit $400 million proved too optimistic, due to lower-than-expected uptake by physicians. In a survey published by Reimbursement Intelligence last August, providers cited questionable clinical utility (versus $93,000 price tag), complexity of treatment regimen (infusion-based, patient-specific “buy and ship” medicine), and uncertainty over reimbursement as critical concerns driving reluctance to use the vaccine.
During the nine months ended September 30, Dendreon reported a net loss of $253 million (excluding non-cash expenses), or $1.73 per share, on aggregate sales of $139.5 million.
Provenge use expected to remain flat
Chief executive officer Mitchell Gold told analysts on the company’s third-quarter earnings call that more doctors were now aware of positive shifts in Medicare and Medicaid coverage decisions – including a reduction in average reimbursement time from 90 days down to 30 days. Dr. Gold stressed, too, that Dendreon was striving to better educate the urology and oncology communities on the acknowledged cost- barrier issue to Provenge usage, articulating that recent data suggested “up to 74 percent of patients had no out-of-pocket costs for Provenge,” and the other 26 percent were being supplemented with assistance programs to “ensure all patients had access to Provenge.”
Best efforts to communicate the cancer vaccine’s cost utility to the urology and oncology communities, however, haven’t translated into greater acceptance of Provenge. Management admitted on the conference call that only modest quarter-on-quarter sequential revenue growth was expected for the rest of the year. Further, no visibility was provided going forward into first-half 2012.
Competitors in the offing
Dr. Susan Slovin, an oncologist with Memorial Sloan-Kettering Cancer Center, speaks to the fears of skittish investors when she promulgated in an interview with Reuters that patients and doctors will likely gravitate away from Provenge toward Johnson & Johnson’s (NYSE:JNJ) newly approved, twice-daily oral Zytiga (abiraterone), which blocks testosterone inside prostatic tumor cells; or, MDV3100 from Medivation (MDVN) and its Japanese partner Astellas Pharma, which has shown impressive results (improved survival) by interfering with the male hormone’s ability to bind to prostate cells, and is being fast-tracked for likely approval in the U.S come 2012.
Healthcare advisory Decision Resources projects worldwide sales of prostate cancer drugs will more than double to $8.9 billion in 2019, from about $4 billion in 2009. Ergo, although Provenge might not live up to initial bullish peak sales forecasts of $2 to $3 billion, contrary to expressed skepticism, a lucrative market still does exist for Dendreon’s proprietary product: Unlike Zytiga and MDV3100, Provenge is approved for use prior to chemotherapy – and, despite the hoopla, mean survival rates (versus control groups) are similar for all three agents (about four months).
Intermediate-term liquidity adequate to handle operating costs
The balance sheet shows the strain of what happens when it takes 15 years to bring a novel, first-in-class cancer treatment to market: An accumulated deficit of $1.6 billion and paltry working capital of just $74.7 million. Problematic – given forecasts of continued flat sales through mid-2012.
Digging deeper into regulatory filings, the 10Q Detective believes Chicken Little running around the company and pointing skyward is premature – the sky “is not falling” at Dendreon – at least not yet. The company can continue to comfortably operate as a “going concern” through 2012:
- Monthly cash burn rate of $35 million is likely to rise in coming months. However, increases in operating costs (such as promotional spending and labor costs) should be mitigated by restructurings (previously announced layoffs) and gains in manufacturing efficiencies (will eventually require sequential improvements in Provenge order flows, particularly at idled manufacturing facilities in New Jersey and Georgia);
- Expected reductions in R&D – abandonment of “go-it-alone” ideology? Albeit company retains global marketing rights, management recently decided to not open its own Provenge manufacturing facility in Europe); and,
- Balance sheet liquidity is stronger than how it looks on paper. For example, $502.6 million in a convertible debenture is classified as a “current liability,” even though the debenture has no holder put options and doesn’t mature until 2016. Simply put, as explained to me by CFO Gregg Schiffman, GAAP accounting requires that the financial instrument be bifurcated into debt and equity portions, with the bulk of the (eventual) liability labeled as short-term debt because the company can force conversion into common stock at any time. (Given strike price is well out-of-the money, I suspect in reality the obligation will remain long-term in nature for several years, at least). Scrub this liability, Dendreon is sitting on a comfortable cash hoard of roughly $568 million!
In response to a question posed by an analyst, CFO Schiffman said once capital costs stabilize – in the opinion of the 10Q Detective, second-half 2012, when the 600 (plus) infusion centers start kicking into gear with new patient starts – cash burn should slow and “eventual cash flow breakeven point is expected to be hit somewhere near $500 million in revenue.”
For speculative investors willing to look ahead, despite dependence on Provenge for 98 percent of product and royalty sales, given Dendreon’s novel and proprietary immune-therapy oncology platform – and an enterprise value of only $1.1 billion – it’s quite possible that a major pharmaceutical or biotechnology company could swoop down and seek to purchase the entire company.
Read More, Part II: Dendreon Buyout, How Imminent?
-David Phillips
Want to Retire Early? Start Here (Sponsor)
Want retirement to come a few years earlier than you’d planned? Or are you ready to retire now, but want an extra set of eyes on your finances?
Now you can speak with up to 3 financial experts in your area for FREE. By simply clicking here you can begin to match with financial professionals who can help you build your plan to retire early. And the best part? The first conversation with them is free.
Click here to match with up to 3 financial pros who would be excited to help you make financial decisions.
Have questions about retirement or personal finance? Email us at [email protected]!
By emailing your questions to 24/7 Wall St., you agree to have them published anonymously on a673b.bigscoots-temp.com.
By submitting your story, you understand and agree that we may use your story, or versions of it, in all media and platforms, including via third parties.
Thank you for reading! Have some feedback for us?
Contact the 24/7 Wall St. editorial team.



