Special Report
Major Milestones in the Race to Vaccinate Every American Against COVID-19
Published:

President Joe Biden just announced the government is working to secure 200 million more COVID-19 vaccines, which, in addition to the doses already distributed and promised, will be enough to fully vaccinate nearly every American.
Two COVID-19 vaccines have been approved and available in the U.S. for almost two months. Several others have been approved and made available around the world. In what has become the biggest vaccination campaign in history, nearly 45 million doses of the vaccines have been distributed around the U.S., and about 10.5 million people have received the two doses needed for full immunization.
Though the vaccine rollout has been bungled, and states are still trying to solve logistical problems that have plagued distribution, the most difficult job of developing a highly efficient vaccine is done. It took about a year to develop, test, and approve several COVID-19 vaccines, which is lightning fast compared to the decade it took to develop vaccines against measles and other diseases.
24/7 Tempo reviewed announcements issued by the Centers for Disease Control and Prevention, the Food and Drug Administration, and several pharmaceutical companies developing COVID-19 vaccines to create a timeline of more than 30 key milestones in the development of COVID-19 vaccines.
Billion-dollar deals were signed between governments and pharmaceutical companies for the development and manufacturing of COVID-19 vaccines, including pre-purchase agreements.
Between safety concerns, unexplained illness of study participants, and technology never before used to develop vaccines, many people were initially reluctant to receive the shot. But some early doubters now show a willingness to get the jab.
The vaccine rollout has brought some easing of movement restrictions, which has been a major source of stress for Americans. The CDC is now saying people no longer have to quarantine, even if exposed to the coronavirus, if two weeks had passed since they received the second dose of a COVID-19 vaccine.
As of Feb. 11, the U.S. surpassed 27 million infections from SARS-CoV-2 and 473,000 deaths. Every state has set priorities about who can get the vaccine first, based on recommendations from the federal government — here are the COVID-19 vaccination priorities in each state.
Click here 38 major milestones in the race to vaccinate every American against COVID-19.
To create a timeline of more than 30 key milestones in the development of COVID-19 vaccines, 24/7 Tempo reviewed press releases and announcements issued by the Centers for Disease Control and Prevention, the Food and Drug Administration, and several pharmaceutical companies developing COVID-19 vaccines. We also reviewed scientific studies’ summaries published in medical journals, including the American Journal of Managed Care.

Jan 11, 2020: China releases genetic data on the novel coronavirus
A month into the unexplained pneumonia outbreak in Wuhan, China, scientists published a draft genome of the novel coronavirus. A DNA sequence of the virus is what researchers need to reverse-engineer a live virus, which can then be helpful in developing antibody tests and starting experiments for possible treatments and vaccines.
[in-text-ad]
Mar 30, 2020: US takes steps to expedite a COVID-19 vaccine
The Department of Health and Human Services starts working with New Jersey-based Janssen Research & Development, part of Johnson & Johnson, and Moderna of Cambridge, Massachusetts, in order to expedite the development and manufacturing of COVID-19 vaccines. The Moderna vaccine candidate uses mRNA, an innovative approach that has not yet been used in approved vaccines against any disease..
Apr 22, 2020: Germany approves its first human trials for COVID-19 vaccine
A clinical trial of a COVID-19 vaccine in Germany is approved. The trial is set to include 200 healthy volunteers between the age of 18 and 55, who will receive different doses of the vaccine, developed by German biotech company BioNTech and its partner, New York-based pharmaceutical giant Pfizer. Like the Moderna vaccine, the Pfizer/BioNTech vaccine candidate uses mRNA technology.

May 15, 2020: US announces Operation Warp Speed
The Trump Administration announces Operation Warp Speed (OWS), a national program to help the development, manufacturing, and distribution of COVID-19 vaccines across the country once they become available.
[in-text-ad-2]

May 21, 2020: US and AstraZeneca reach vaccine deal
The federal government and British-Swedish drugmaker AstraZeneca announce a collaboration to speed the development of a COVID-19 vaccine. HHS says it expects the first doses — at least 300 million of them — to be available as early as October 2020. Phase 3 clinical trials are set to be conducted during the summer.

Jun 11, 2020: Moderna to begin Phase 3 trial of its COVID-19 vaccine
Moderna announces that the company would enter the third stage of its clinical trial, required for regulatory approval, in July with 30,000 participants. The company says it can produce between 500 million to 1 billion doses by the beginning of 2021.
[in-text-ad]

Jul 14, 2020: Moderna vaccine shows a positive immune response
The 45 patients who were given the Moderna’s COVID-19 vaccine produced antibodies that could neutralize the novel coronavirus that causes the disease, according to preliminary data from an early-stage trial. Some minor side effects such as chills and pain at the injection site have been observed in more than half of the volunteers.
Jul 21, 2020: Vaccines from AstraZeneca, CanSino Biologics show promising results
An experimental vaccine from AstraZeneca in collaboration with Oxford Vaccine Group and another from CanSino Biologics, a Chinese vaccine company, show promising results in providing protection against COVID-19. The Oxford/AstraZeneca vaccine generated robust immune responses against the virus in all participants in a Phase 1/2 trial, according to results published in The Lancet.
The CanSino vaccine was effective in 95% of patients who were still showing immune responses 28 days after vaccination, according to Phase 2 results also published in The Lancet.

Jul 22, 2020: US reaches vaccine distribution agreement with Pfizer and BioNTech
HHS and the Department of Defense announce a partnership with Pfizer and BioNTech, which commit to deliver 100 million doses of their COVID-19 vaccine candidate by December 2020. If approved by the FDA, the companies will receive $1.95 billion for manufacturing of the vaccine, which will be available for free to the general public.
[in-text-ad-2]
Jul 27, 2020: Moderna begins Phase 3 COVID-19 vaccine trials in the US
The first Phase 3 clinical trial of the coronavirus vaccine developed by biotechnology company Moderna and the National Institute of Allergy and Infectious Diseases begins in the United States. The trial is to be conducted at nearly 100 research sites and aims to enroll approximately 30,000 adults who do not have COVID-19.

Aug 3, 2020: US to pay Sanofi, GlaxoSmithKline $2 billion for vaccine
As the U.S. experiences a second surge of the pandemic, the federal government announces a deal to pay $2.1 billion to GlaxoSmithKline and Sanofi Pasteur, a British and French pharmaceutical companies, to develop, manufacture, and scale up delivery of a COVID-19 vaccine.
[in-text-ad]
Aug 11, 2020: Russia first to approve a COVID-19 vaccine
Russia announces that its health regulator has approved a coronavirus vaccine for widespread use, becoming the first country in the world to do so. The news, however, is met with much skepticism by scientists across the world who accuse Russia of skipping large trials aimed to test the vaccine’s safety and efficacy.

Aug 11, 2020: US reaches deal with Moderna
The U.S. government agrees to pay Moderna $1.53 billion for 100 million doses of its vaccine candidate, or an average of $15 per-dose. The vaccine is still under investigation in Phase 3 trial, with the results expected around October. Moderna has already received almost $1 billion from the government for developing the vaccine.
Aug 12, 2020: Pfizer publishes initial Phase 1/2 clinical trial data
According to published results, the Pfizer/BioNTech vaccine produced mild side effects such as mild fever and body aches in fewer than 20% of participants. Older participants tended to have less reaction than younger ones. However, lower neutralizing antibody response was observed in the older participants.
[in-text-ad-2]

Sep 1, 2020: US rejects WHO global COVID-19 vaccine initiative
The U.S. government says it will not participate in an initiative by the World Health Organization to develop, make, and distribute a COVID-19 vaccine. The initiative, coined COVAX, was launched so that an eventual vaccine can be distributed evenly to at least 20% of the population of the 172 participating nations.

Sep 3, 2020: Sanofi, GSK begin human COVID-19 vaccine trials
Sanofi and GSK start a human clinical trial of their protein-based vaccine, which uses the same protein-based technology as one of Sanofi’s flu vaccines and is combined with a vaccine developed by GSK. About 440 adults in the U.S. will participate.
[in-text-ad]

Sep 8, 2020: AstraZeneca pauses COVID-19 vaccine trial after a volunteer becomes sick
AstraZeneca pauses its COVID-19 vaccine trial after unexplained illness in one participant. The move is just a precaution and a routine action, according to the drug giant. At this time, the AstraZeneca vaccine is one of three COVID-19 vaccines in late-stage, Phase 3 trials in the U.S.
Sep 14, 2020: Pfizer, BioNTech expand Phase 3 trial
After initially recruiting participants, Pfizer and BioNTech announce they will expand the Phase 3 trial of their COVID-19 vaccine to 44,000. The goal is to increase data on safety and efficacy as well as to include a more diverse population, including younger people, minorities, and those with pre-existing conditions.
Sep 16, 2020: US releases vaccine distribution plan
A vaccine distribution plan, devised by HHS and the DOD, aims to make COVID-19 vaccines free for all Americans, with vaccine roll out set for January 2021. Once a vaccine is authorized, 6.6 million kits of supplies will be distributed to administer up to 660 million doses of the vaccine. No decision on who will be the first to receive the vaccine is made yet.
[in-text-ad-2]

Sep 22, 2020: Johnson & Johnson begins Phase 3 vaccine trial
Johnson & Johnson begins a large Phase 3 clinical trial of its COVID-19 vaccine candidate. With 60,000 participants, the trial is the largest of any COVID-19 vaccine being tested at the time. The vaccine does not need to be frozen and may require only one dose instead of two.
Sep 29, 2020: Moderna’s COVID-19 vaccine shows acceptable safety
The Moderna vaccine, which is being developed in partnership with the National Institutes of Health, has been tested in 40 adults 56 and older. The vaccine is found to safely trigger an immune response in that age group, according to the preliminary findings.
[in-text-ad]
Oct 12, 2020: Johnson & Johnson pauses COVID-19 vaccine trials after a participant contracts an unexplained illness
Johnson & Johnson pauses its COVID-19 vaccine trial over a participant’s “unexplained illness,” though it is unclear whether the patient was receiving the vaccine or the placebo. The study includes 60,000 volunteers.
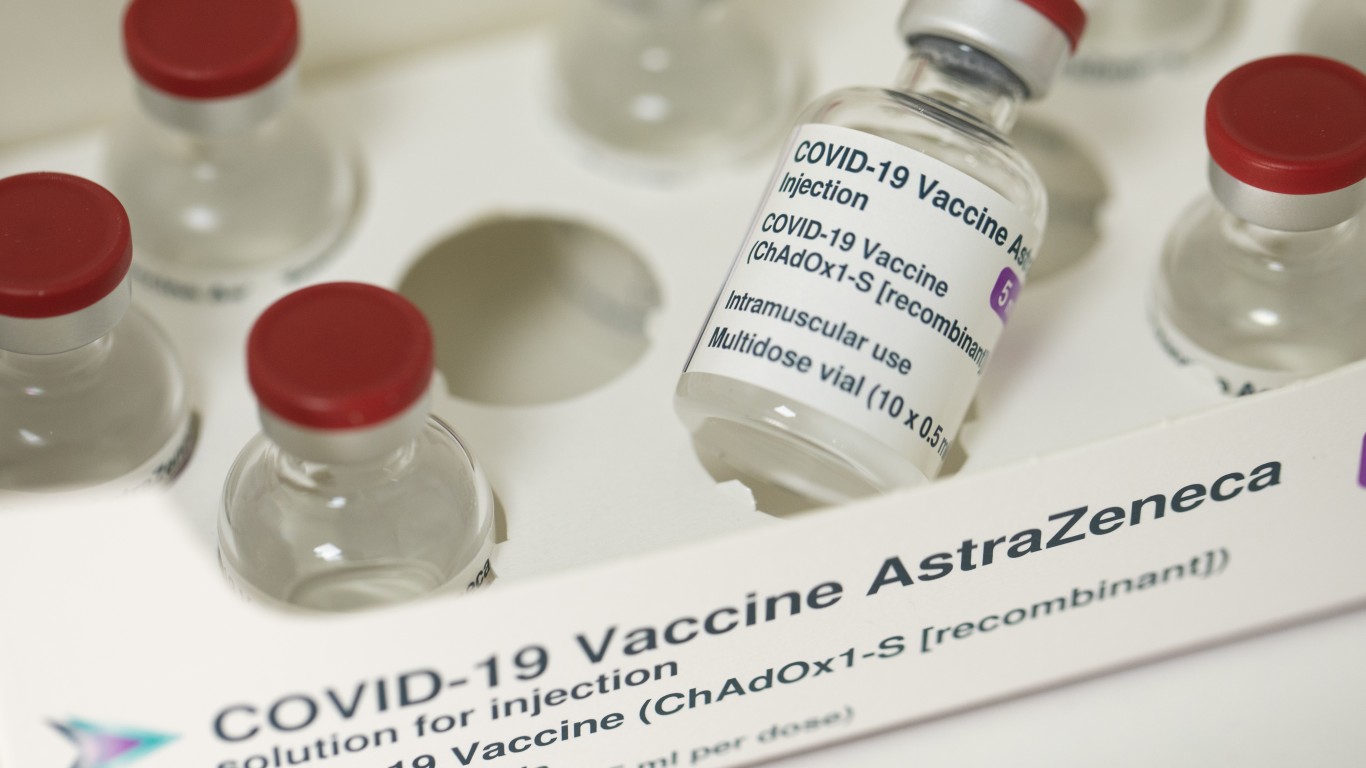
Oct 23, 2020: AstraZeneca and Johnson & Johnson restart COVID-19 vaccine trials
AstraZeneca and Johnson & Johnson announce plans to restart clinical trials for their respective COVID-19 vaccine candidates after they both stopped due a participant falling sick. Johnson & Johnson’s trial stalled on Oct. 12, and AstraZeneca halted its study on Sept. 6. An independent monitoring committee determined that the trial for the AstraZeneca vaccine candidate was safe to continue.
Nov 16, 2020: Moderna reveals vaccine is 95% effective
Moderna announces that its experimental vaccine is 94.5% effective in preventing COVID-19. Like Pfizer’s vaccine, the Moderna vaccine uses mRNA, an innovative approach that has not yet been used in approved vaccines against any disease.
[in-text-ad-2]

Nov 18, 2020: Pfizer-BioNTech vaccine shows 95% efficacy
The results of a nearly 44,000-person trial demonstrate that the Pfizer-BioNTech COVID-19 vaccine is 95% effective, making it as effective as vaccines for shingles and measles. Pfizer also announces that it will seek FDA approval soon so distribution of the vaccine can start by the end of 2020.

Nov 23, 2020: AstraZeneca reports vaccine is 90% effective
When AstraZeneca’s COVID-19 vaccine is administered as a half dose followed by a full dose at least a month later, the vaccine can be up to 90% effective (a discovery made by accident). The drugmaker says it can have as many as 200 million doses by the end of 2020 and 700 million by the end of the first quarter of 2021.
[in-text-ad]
Dec 7, 2020: UK first to roll out the Pfizer-BioNTech vaccine
In the U.K., a 90-year-old woman becomes the first person in the world to be given the Pfizer-BioNTech COVID-19 vaccine as part of a mass vaccination program. People who are 80 and over and who are either in a hospital or are being discharged after a hospital stay are the first to receive the vaccine in the U.K.

Dec 11, 2020: FDA issues Emergency Use Authorization for Pfizer-BioNTech vaccine
The FDA agrees to issue an Emergency Use Authorization (EUA) for the Pfizer-BioNTech vaccine, allowing shipments to begin. Vaccinations of health care workers are to begin within days.
Dec 14, 2020: US begins vaccinating population
The Pfizer-BioNTech vaccine against COVID-19, the first federally approved coronavirus vaccine in the U.S., is administered to health care workers and staff at nursing homes nationwide.
[in-text-ad-2]
Dec 15, 2020: FDA finds Moderna vaccine safe
The FDA confirms the effectiveness and safety of the Moderna COVID-19 vaccine, clearing a path to authorization. The potential second COVID-19 vaccine is found to be 95% effective.
Dec 18, 2020: FDA issues Emergency Use Authorization for Moderna COVID-19 vaccine
The FDA issues an EUA for the Moderna COVID-19 vaccine, allowing shipments to begin. The vaccine has been deemed safe for people 18 and older. (The Pfizer-BioNtech vaccine can be used in people 16 years and older.)
[in-text-ad]

Dec 21, 2020: Dec 21: States receive first Moderna vaccines
A week after the first doses of the Pfizer-BioNTech coronavirus vaccine are administered in the U.S., a new batch of vaccines, made by Moderna, fan out across the country. Moderna’s vaccine rollout is four times larger than Pfizer’s.
Dec 23, 2020: US buys more Pfizer-BioNTech COVID-19 vaccine
The U.S. government announces it will buy an additional 100 million doses of Pfizer-BioNTech’s vaccine. At least 70% of the doses must be delivered by June 30, 2021, and all of them no later than July 31, 2021.
Dec 30, 2020: UK approves Emergency Authorization for the AstraZeneca-Oxford COVID-19 vaccine
The U.K. becomes the first country to provide an emergency authorization of the AstraZeneca COVID-19 vaccine for people 18 and older. The goal is to vaccinate 2 million people a week in the country.
[in-text-ad-2]
Dec 30, 2020:UK to begin giving as many people as possible a first vaccine dose
About two weeks after the announcement of a new, more contagious variant of the coronavirus, the British government decides to begin giving as many people as possible a first vaccine dose rather than holding back supplies for the second shots as a way to speed up vaccinations.

Dec 31, 2020: US falls short of goal to vaccinate 20 million Americans
The federal government does not meet its goal to administer 20 million coronavirus vaccines by year-end due to logistical problems and underfunded health departments. By Dec. 31, 2.8 million people receive the first of two doses needed to provide immunity to the virus, according to the CDC.
[in-text-ad]
Jan 4, 2021: UK rolls out Oxford-AstraZeneca vaccine
The U.K. starts rolling out 500,000 doses of the coronavirus vaccine developed by AstraZeneca and the University of Oxford, a week after the shot was approved. The U.K. government has an agreement for 100 million doses, enough for 50 million people.
Feb 11, 2021: US reaches deal for 200 million vaccines doses
The U.S. government announces it will buy 200 million more doses of the COVID-19 vaccines that have so far been approved for use in the country, a step that may provide enough doses to fully inoculate almost every American eligible for the vaccine by the end of the summer.
Credit card companies are handing out rewards and benefits to win the best customers. A good cash back card can be worth thousands of dollars a year in free money, not to mention other perks like travel, insurance, and access to fancy lounges. See our top picks for the best credit cards today. You won’t want to miss some of these offers.
Flywheel Publishing has partnered with CardRatings for our coverage of credit card products. Flywheel Publishing and CardRatings may receive a commission from card issuers.
Thank you for reading! Have some feedback for us?
Contact the 24/7 Wall St. editorial team.